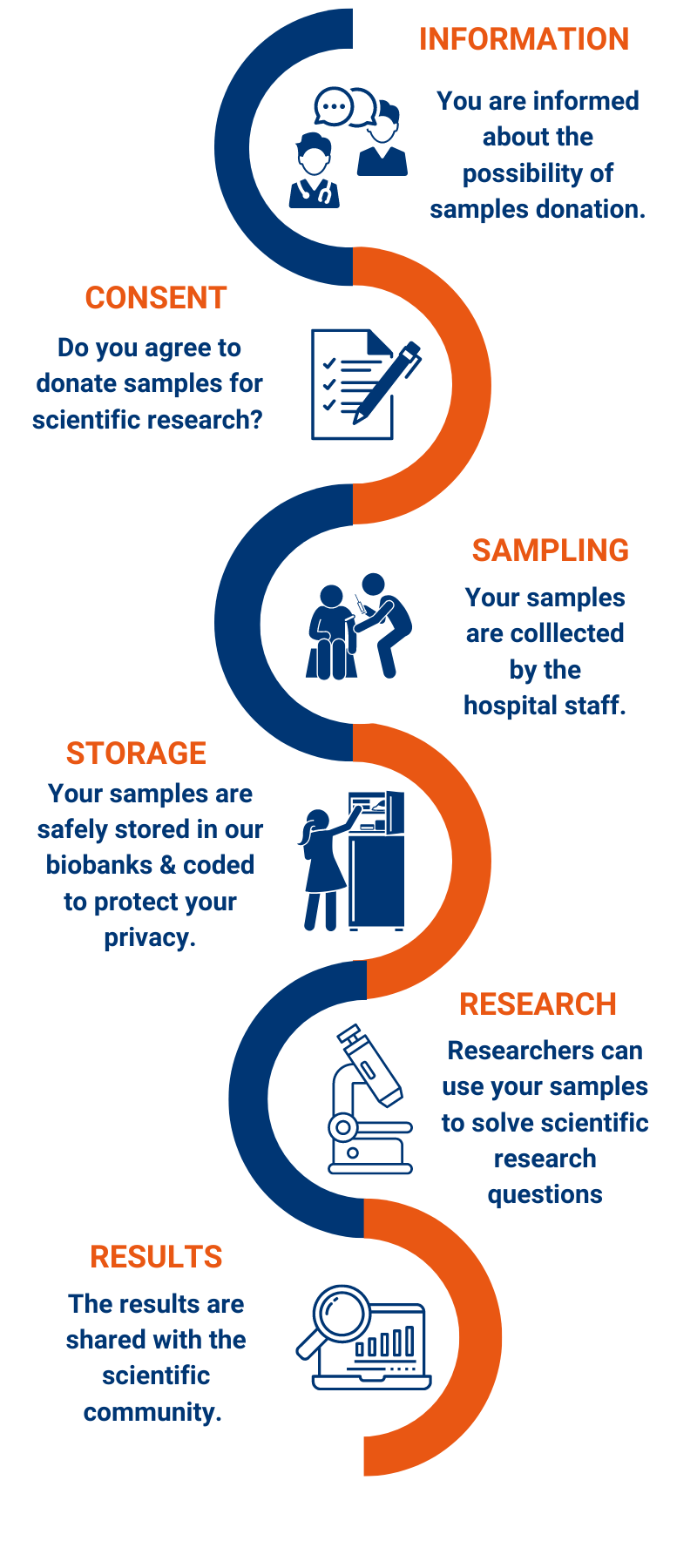
What happens with your samples?
What happens with your samples?
Frequently asked questions
Biobank
A biobank is a research infrastructure which…
– obtains
– treats
– stores
– makes available
… human body material (and related data) for scientific research
Which samples are collected in the biobank?
All human body material collected for scientific research needs to be stored in a biobank.
The type of material collected depends on the needs of the scientific research (e.g. tissue, blood, stool, saliva, urine, tooth, semen, DNA, RNA, cells, tear drops, ….).
Which samples are not collected in the biobank?
Samples collected for treatment of patients (cell therapy, transplantation, …). These samples are stored in the tissue bank of the hospital.
BBMRI.be
BBMRI.be is a network of Belgian biobanks and is part of the European biobank infrastructure BBMRI-ERIC.
All biobanks in our network are approved by the Federal Authorities and Ethical Committees and work according to the principles of the Belgian Biobank Law.
What do we do in this network?
Within the BBMRI.be network, we exchange expertise between the different biobanks.
We inform the biobanks about the new guidelines and regulations for biobanks and help them to implement this in their biobank.
Which biobanks are part of our network
Most of our biobanks are linked to (university) hospitals based in Belgium and store samples of patients treated in these hospitals.
Our network also has some biobanks that are linked to scientific institutes /research organisations which collect and store samples to support their scientific developments.
Presumed consent
Human body material that is left-over after a diagnostic procedure or surgery, can be used for scientific research.
This material, together with the associated coded data, is stored in the biobank of the hospital for future scientific research under the supervision of the ethical committee of the hospital.
Your hospital should inform you about this policy of presumed consent when you are admitted to the hospital.
If you do not agree with this, you or your representative have the right to refuse this use at any time.
You can communicate this refusal to the healthcare professionals under whose responsibility the removal of the body material falls.
This refusal (opt-out) will then be added to your medical file and will assure that your body material will not be used for scientific research.
Informed consent
 Many hospitals, actively contribute to the continuous improvement of healthcare by conducting scientific research.
Many hospitals, actively contribute to the continuous improvement of healthcare by conducting scientific research.
If at the department where you are admitted, a specific study is ongoing, you may be asked to participate in it. You will have full freedom to decide whether or not you wish to participate.
If you agree to participate in the study, you will be asked to sign an informed consent.
In this informed consent, all information can be found on the type of additional samples/data that will be collected and what research will be performed on these samples.
Samples
 A biobank can store all kind of human body material (tissue, blood, stool, saliva, urine, tooth, semen,DNA, RNA, cells, tear drops, ….).
A biobank can store all kind of human body material (tissue, blood, stool, saliva, urine, tooth, semen,DNA, RNA, cells, tear drops, ….).
The type of samples that are collected depend on which material is needed for research.The samples are collected and stored according to strict guidelines to make sure that all samples are of high quality and can be used for scientific research.
Data
The data will always be coded to protect the privacy of the patient.
Research after presumed consent
Left-over material that is no longer needed for the diagnosis of the patient, can be stored in a biobank if the donor does not object.
At the time when samples are collected, it is not yet clear for which scientific research these will be used.
Researchers can use these samples for their research project if their research project is approved by the ethical committee
Research for a specific study
Samples collected for a specific project, not as part of usual care, and after informed consent, will in first instance be used for this research project.
If there is material left afterwards, these samples can be used for other research purposes after approval by the donor and an ethical committee.