Frequently asked questions
The central BBMRI.be support in the B3-ISO project to reach levels Q1, Q2, and Q3 are financed in the project, the initial assessment form Belac to reach Q4 is not financed by the B3-ISO project.
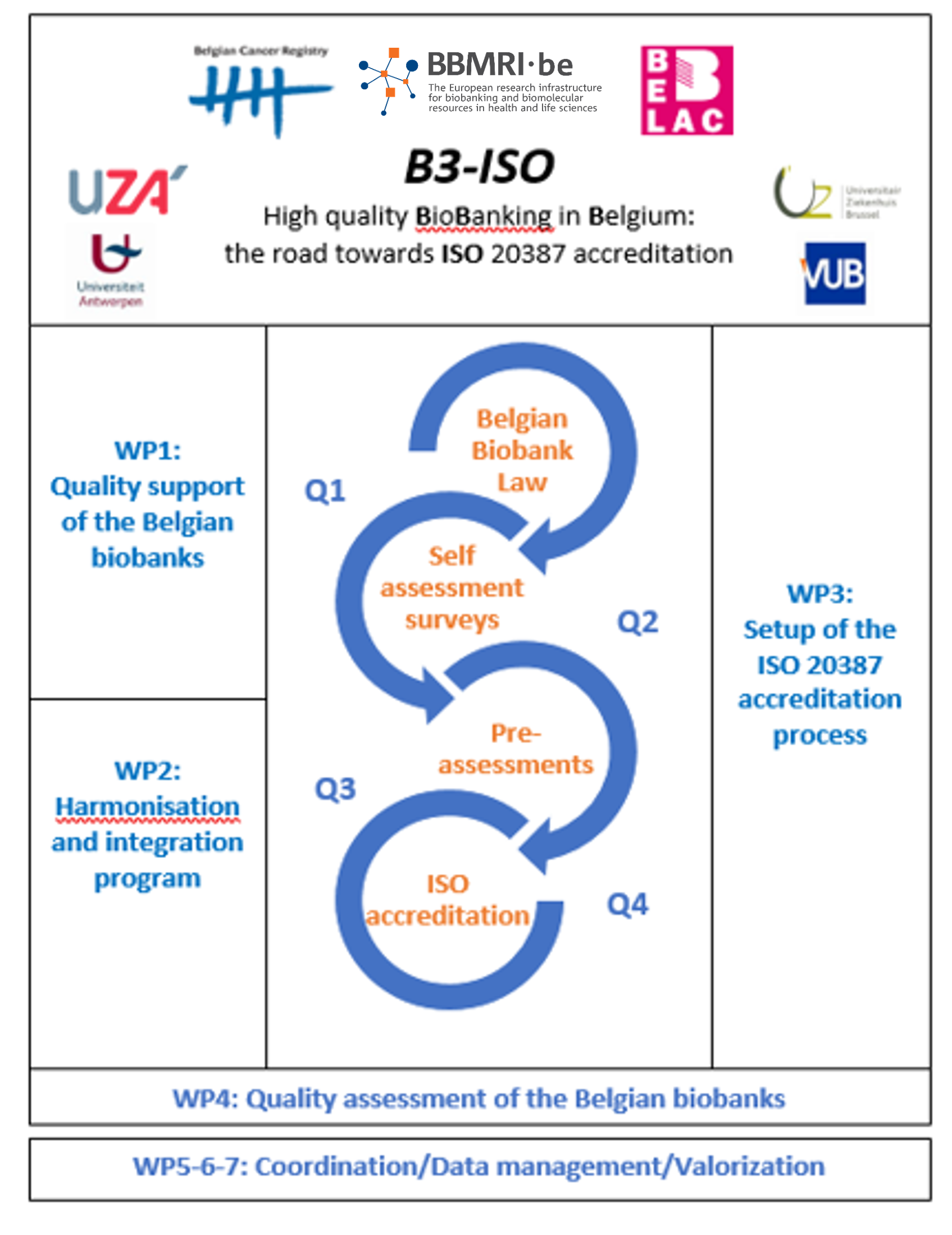
The leadership in the work packages is as follows:
WP1: Quality support of the Belgian biobanks: WP leader: BCR/BBMRI.be
WP2: Harmonisation & integration program: WP leader: UZBrussel & UZA
WP3: Setup of the ISO 20387 accreditation process: WP leader: BELAC
WP4: Quality assessment of the Belgian biobanks: WP leader: BCR/BBMRI.be & BELAC
WP5-6-7: Coordination/datamanagement/valorisation: WP leader: BCR/BBMRI.be
BBMRI.be support in the B3-ISO project to reach levels Q1, Q2, and Q3 are financed in the project, but the initial accreditation assessment to reach Q4 is not financed by the B3-ISO project.
Within the B3-ISO project, we will support the biobanks in the road towards ISO 20387 accreditation by providing:
- templates/ guidelines & webinars to facilitate obtaining the different Quality levels of BBMRI.be (Q1-Q4)
- a continously updated list of FAQ on the website
- a internal audit organized by BBMRI.be/BBMRI-ERIC
- a pre-assessment by BELAC in preparation of the official ISO 20387 audit
Next to the Quality related support, several other templates will be developed in collaboration with the other Working Groups of BBMRI.be.
This includes templates for informed consent, harmonized cost calculation, harmonized datasets, …
All developed documents will be available on the intranet of BBMRI.be. The public available documents will also be posted on the B3-ISO page of the website.
The B3-ISO scheduled external audits can be in the language of your choice: English, French or Dutch. This choice is to be agreed upon with the external auditor. We will make efforts to offer audits in the biobank’s language of choice.
These are two separate tools
The BBMRI-ERIC Self-Assessment Survey (SAS) is developed by BBMRI-ERIC. This tool is available to members of BBMRI-ERIC for free, and non BBMRI-ERIC members can take if after paying a fee. It is developed to check how biobanks are performing regarding the ISO norms. This is the questionnaire that is used in the B3-ISO project and addressed in a B3-ISO webinar.
The ISBER BAT (Biobank Assessment Tool) is a questionnaire developed by the International Society for Biological and Environmental Repositories (ISBER) and allows a biobanks to check how they are performing regarding the ISBER Best Practices. Next to that, it provides some high-level references to applicable ISO20387 clauses, but not all content from ISO20387 is covered by the questions of this survey. Along with the certificate and the score in %, you get a report that can be imported in excel for further use. More info can be found at https://www.isber.org/general/custom.asp?page=BAT. This tool is not related to the B3-ISO project.
All BELAC prices are published in BELAC 7-01: BELAC: General and practical information (https://economie.fgov.be/en/publication/series-belac-7-xx-information).
Dossier cost: non-recurrent, to be payed upon submission of accreditation-request
Yearly fee during accreditation
Audits: rate is calculated per hour. The price will depend on the scope and the type of audit.
Estimation for pre-assessment: 17 hours
Estimation for initial audit with limited scope: 54 hours in case of 1 main auditor & 1 technical auditor.
The B3-ISO project is a project funded within the ESFRI-FED call of BELSPO to support the participation of Belgian networks in ESFRI distributed and virtual research infrastructures.
The general aim of our project is to harmonize and enhance the quality management activities of the BBMRI.be biobanks.
To achieve this, a stepwise quality improvement program will be established to guide the biobanks in the road towards ISO 20387 accreditation.
In parallel, the Belgian accreditation body BELAC will set up and implement an ISO 20387 accreditation program.
To valorize the results obtained in this project, a harmonization and integration program will be set up developing harmonised minimal datasets and aiming toward simplification of inter-biobank operation on legal and financial aspects.
ISO defines uses specific language to address this. (see https://www.iso.org/foreword-supplementary-information.html)
“shall” indicates a requirement
“should” indicates a recommendation
“may” is used to indicate that something is permitted
“can” is used to indicate that something is possible, for example, that an organization or individual is able to do something
Requirements and recommendations are defined as follows (ISO/IEC Directives, Part 2, 2021, 3.3.3-3.3.4) :
A requirement is an “expression, in the content of a document, that conveys objectively verifiable criteria to be fulfilled and from which no deviation is permitted if conformance with the document is to be claimed.”
A recommendation is an “expression, in the content of a document, that conveys a suggested possible choice or course of action deemed to be particularly suitable without necessarily mentioning or excluding others.”
A lead auditor focuses on general aspects of the overarching QMS (CA, personnel,…).
A technical auditor focuses on very specific tasks (e.g., tests in ISO17025). The specifics are not yet clear for ISO20387 technical auditors, as this will probably depend on the scope of the audited biobank
This could be, and you could do a validation study for this. We refer to the following sources:
Review on long-term storage temperatures: https://www.liebertpub.com/doi/10.1089/bio.2013.0084
Lukasz Kozera BBMRI-ERIC webinar series on pre-analytical quality: https://www.bbmri-eric.eu/services/e-learning/
BBMRI.de white paper: https://www.bbmri.de/fileadmin/user_upload/PDFs/GBN_Recommendations_Energy_Saving_-80-ULT_Nov2022.pdf
Yes, the Quality Manual, SOP or other documented information that you use in your biobank can be in the language of your choice. Having this information available in the mother tongue of the employers can surely be beneficial for their implementation, this is a strong argument not to choose English.
- 4.1.4 Information relevant to biobank activities, processes and procedures shall be documented in a comprehensible format. What does this actually mean? It’s very vague to me. Does this adress procedures we need to provide to our personnel members, or does it also concern communication to other stakeholders such as donors?
The biobank has to document all relevant processes and their interdependencies. (adapted from the German Quality Manual)
What is relevant are the critical biobanking activities, and that is something that the biobank can decide themselves. ISO20387 annex A needs to be implmented for the critical activities of the biobank, and annex B can provide guidance. - 4.1.5 The documentation shall include relevant information generated from procedures pertaining to the quality management system (see Clause 8) as well as the management of facilities/dedicated areas. What does this actually mean? It’s very vague to me.
Does this adress procedures we need to provide to our personnel members, or does it also concern communication to other stakeholders such as donors?
The biobank has to document all relevant processes and their interdependencies, including how you manage the rooms used in biobanking activities (adapted from the German Quality Manual)
What is relevant are the critical biobanking activities, and that is something that the biobank can decide themselves. ISO20387 annex A needs to be implmented for the critical activities of the biobank, and annex B can provide guidance. - 4.1.7 The biobank should document the identity of personnel performing activities encompassing procedures as referred to in 4.1.1 Is this applicable to all processes performed; should we register who’s handling the sample at each seperate step in the biobanking process?
The biobank has to document the tasks and responsibilities of internal staff and external users regarding all critical biobanking activities.
The instructions are binding for all biobank employees. The requirements for externally provided services also need to be described, but as the biobank doesn’t have them under direct control, the instructions are not binding for external personnel. (adapted from the German Quality Manual) - On page 32 an annex B: B.2 are listed: Acquisition, collection method, method of sampling.
What does method of sampling mean here? Is this the same as the ‘type of collection’ data field in SPREC? It’s also not entirely clear to me why this data field is improtant.
‘Specific properties’ is added to Annex A (A2g) because specific extra information can be relevant for specific sample types. It’s added there as a reminder: if there would be relevant info with regards to specific properties, then you need to record that info. - On page 32 an annex B: B.2 are listed: Acquisition, specific properties – infectiousness — biosafety information radioactivity/radiation – transgenic, chimera, genetically modified etc
Do we need to define this of each study where this is applicable? Are there other things to keep in mind that could fall in the categogy ‘specific properties’?
‘Specific properties’ is added to Annex A (A2g) because specific extra information can be relevant for specific sample types. It’s added there as a reminder: if there would be relevant info with regards to specific properties, then you need to record that info.
The B3-ISO ISO20387 Workbook and associated documents are only guidance for you information. Their use is not mandatory.
Funding is only provided for BBMRI.be members.
If additional biobanks become BBMRI.be members over time, they too will have access to support for reaching levels Q2 to Q4.
Certain types of support, such as FAQ will be available open source.
Acquisition, storage and distribution are all obligatory activities in the BELAC ISO20387 accreditation scope. Therefore it’s not possible to receive an ISO20387 accreditation if your biobank only includes storage in its accreditation scope, and excludes acquisition and distribution.
However, the activities of collection, preparation, testing and preservation are not obligatory activities in the BELAC ISO20387 accreditation scope. Your biobank can still apply for ISO20387 accrediation when it excludes one or more of these activities from their accreditation scope.
Biobanks don’t have to use a quality manual, SOPS or WDs. They can structure their documented information as is most convenient for them. When they wish to attain ISO20387 accreditation, this needs to be conform the requirements.
The audit is performed by default in your own language (French, Dutch, German). This can differ from the language of your documented information. It can also be requested to have an audit performed in English.
General requirements clauses address is a very broad way all aspects of biobank management and activities, such as the general principles of quality documentation, impartiality and confidentiality.
Structural requirements clauses address high-level management aspects such as description of the organizational context, definition of the scope of the quality management system & quality policy, and description of leadership, roles and authorities.
ISO TC212 (Clinical laboratory and IVD test systems) published Technical Specifications for pre-examination processes for a broad range of matrices and uses.
The most recent versions can be found at https://www.iso.org/committee/54916/x/catalogue/p/1/u/0/w/0/d/0.
Documents still under development are listed at https://www.iso.org/committee/54916/x/catalogue/p/0/u/1/w/0/d/0
The cost of a pre-assessment audit is included in B3-ISO budget for 20 biobanks. A pre-assessment audit is not mandatory and can be skipped. Contact B3-ISO@kankerregister.be if you wish to discuss the possibility to relocate the budget to partially finance the assessment audit.
It’s up to the biobank to define the scope of their activities and quality objectives. If you exclude certain activities or collections out of your scope of accreditation, you cannot declare that your accreditation certificate is applicable to those activities or collections.
Yes, because adherence to the applicable laws is a prerequisite for BBMRI.be membership, and BBMRI.be membership is a prerequisite for funded participation in the B3-ISO project.